IRMS Technique
Introduction
Analytical science, currently applied in support of a justice system, can establish a degree of resemblance between one substance and another by means of identifying the constituent elements, cations, anions, and functional groups, and by elucidating structure. Should two substances correspond, it may be concluded that they are chemically the same substance.
However, it has always been a defence that although the two substances in question are chemically the same, they have a different source. The analytical technique stable isotope ratio mass spectrometry (IRMS) now allows this contention to be tested and potentially provides resolution.
Initial research has confirmed the potential probative power of the data provided by this analytical technique. The stable isotope compositions of elements that are part of a substance are a function of the origin and history of that substance. That is, two substances which are chemically the same may have different stable isotope compositions if either their origin and/or history differ. This could remove the defence of the same substance being from different sources and thus be a significant advance in forensic science, crime detection and reduction.
This technique would significantly increase the probative power of analytical results for drugs, explosives, fibres, textiles, glass, paints, papers, inks, plastics, adhesives, etc.
Stable isotopes
Isotopes are defined as atoms whose nuclei contain the same number of protons, but a different number of neutrons. All but 12 elements exist as mixtures of isotopes. The proportions of these isotopes can vary greatly. An example of the stable isotopes of hydrogen, hydrogen-1 and hydrogen-2 (known as deuterium), is shown below:
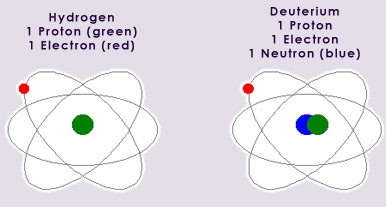
The proportions of stable isotopes listed below are approximately the mean values found naturally throughout Earth. The small variations in these values are the way of characterising materials. The units in the following table are atom % (the particular concentration of that isotope).
| Element | Major Isotope | Largest Trace Isotope | Trace Isotope ppm (atoms/106 atoms of major isotope) |
|---|---|---|---|
| Hydrogen | 1H | 2H | 158 |
| Carbon | 12C | 13C | 11,000 |
| Nitrogen | 14N | 15N | 3,700 |
| Oxygen | 16O | 18O | 2,000 |
| Sulphur | 32S | 34S | 42,000 |
| Chlorine | 35Cl | 37Cl | 245,000 |
| Bromine | 79Br | 13Br | 493,000 |
Isotope Ratio Mass Spectrometer
A mass spectrometer is an instrument that separates charged molecules by mass. Various types of methods can be employed to perform this.
An isotope ratio mass spectrometer works on this principle, but unlike other conventional mass spectrometers it has been specifically designed to measure the proportions of particular isotopes.
An isotope ratio mass spectrometer will be much more precise, but much less sensitive, than other mass spectrometers. The mass spectrometers used for isotopic analysis generally comprise three basic sections: an ion source, a mass analyser, and an ion collection assembly.
In the simplified illustration, gaseous molecules are introduced into the ionisation chamber where interaction with a focused electron beam causes electrons to be stripped from the molecules, resulting in the formation of positive ions.
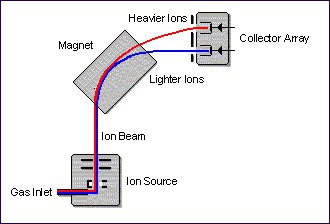
The ions are then accelerated out of the chamber, down a flight tube that is placed between the poles of an electromagnet. Here, they are separated according to their mass-to-charge ratio (m/z).
The ions are typically collected by a simple collector array consisting of three Faraday cup collectors. In order to carry out IRMS analysis, only pure gases, e.g. N2, CO2, or pure gas contained within a carrier gas, can be analysed.
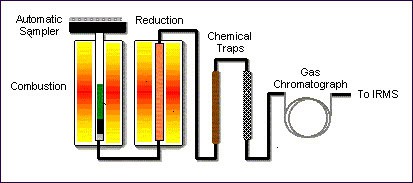
Continuous flow elemental analysis (CF-EA)
Sample preparation, and subsequent analysis, is carried out on-line in a continuous flow of helium. An elemental analyser is an automated sample preparation instrument in which samples are converted into pure gases via combustion, reduction, and pyrolysis reactions in the presence of catalysts.
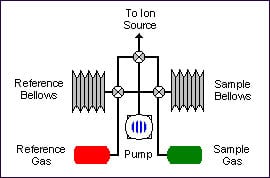
Dual inlet
Samples are prepared off-line. The pure gas is admitted into the isotope ratio mass spectrometer by a variable volume, i.e. bellows. A reference gas is also admitted into the spectrometer via a bellows system.
Other methods
Other methods of introducing samples to an isotope ratio mass spectrometer exist. One of these involves the attachment of a gas chromatograph. This technique is ideal for analysing samples that are complex mixtures of organic materials held in a solvent.
The separation of the compound that is to be analysed occurs during gas chromatography (GC) before being converted to a pure gas by a high temperature reactor.
Information previously obtained from Iso-Analytical Limited.
Newsletter Signup
Enter your email below to sign up to our newsletter.
We keep your data private and share only to provide this service. Read our full Privacy Policy.
